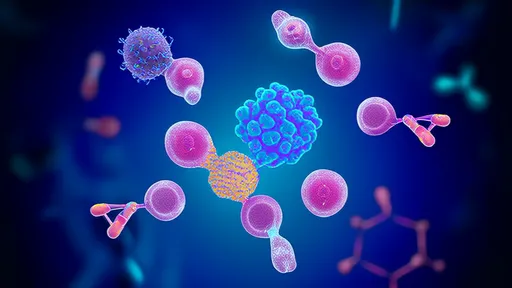
In a landmark development that could redefine how we approach aging and age-related diseases, the field of senolytics has achieved a significant breakthrough in targeted clearance of senescent cells. These so-called "zombie cells," which accumulate with age and refuse to die, have long been implicated in driving inflammation, tissue dysfunction, and a host of chronic conditions. The latest advancements are not merely incremental improvements but represent a paradigm shift in precision and efficacy, moving us closer to therapies that could potentially delay, prevent, or alleviate multiple ailments of aging simultaneously.
The core challenge in senolytic therapy has always been specificity. Senescent cells play complex roles; they are not universally detrimental and are involved in vital processes like wound healing and tumor suppression. Therefore, indiscriminate destruction is not a viable option. Earlier generations of senolytics, while promising, often faced limitations related to off-target effects and manageable but concerning side effects. The new wave of technologies, however, leverages a much more sophisticated understanding of the unique biological signatures that distinguish a senescent cell from its healthy neighbors.
Researchers are now pioneering drugs that exploit these specific vulnerabilities. One of the most promising strategies involves targeting the senescence-associated secretory phenotype (SASP). Senescent cells are notoriously chatty, releasing a potent cocktail of inflammatory cytokines, growth factors, and proteases that poison the local tissue environment. The new class of drugs aims to disrupt the very machinery that produces this harmful signals, effectively putting a muzzle on these cells before triggering their apoptosis, or programmed cell death.
Another frontier involves the use of antibody-based therapies and proteolysis-targeting chimeras (PROTACs). These are highly precise biological missiles designed to seek out and destroy. Antibodies can be engineered to bind exclusively to proteins that are highly expressed on the surface of senescent cells. Once bound, they can flag the cell for destruction by the body's own immune system or deliver a cytotoxic payload directly to its doorstep. PROTACs offer an even more elegant solution by hijacking the cell's own waste-disposal system to degrade specific proteins essential for the senescent cell's survival.
Perhaps the most cutting-edge approach involves gene therapy and RNA interference techniques. The idea here is to design genetic circuits that are only activated within the context of a senescent cell. Once activated, these circuits could express a gene that instructs the cell to self-destruct, all while leaving healthy cells completely untouched. This level of precision, moving from pharmacological to genetic targeting, marks a quantum leap in the potential safety and long-term effectiveness of senolytic interventions.
The implications of these technological leaps are profound. In preclinical models, these next-generation senolytics have demonstrated a remarkable ability to rejuvenate tissues. Aged mice treated with these targeted therapies showed improved cardiovascular function, enhanced cognitive performance, increased physical endurance, and a general reduction in biomarkers of aging. The clearance of senescent cells from arthritic joints reduced pain and stimulated the regeneration of cartilage, suggesting a potential cure for osteoarthritis rather than mere symptom management.
Beyond single organs, the systemic nature of senescence means these drugs could tackle multiple age-related diseases at once. A patient taking a senolytic for kidney fibrosis might also experience improved lung function and a lower risk of cardiovascular events. This holistic approach stands in stark contrast to the current model of medicine, which often treats each age-related condition in isolation with a different pill, leading to polypharmacy and its associated risks.
Of course, the path from laboratory breakthrough to widely available treatment is long and fraught with challenges. Rigorous clinical trials are needed to confirm efficacy and safety in humans over the long term. Questions about treatment regimens—whether it will be a periodic cleanse or a continuous therapy—remain unanswered. Furthermore, the economic and ethical dimensions of a powerful anti-aging therapy will undoubtedly spark intense debate within healthcare systems and society at large.
Despite these hurdles, the momentum is undeniable. Major pharmaceutical companies and biotech startups are investing heavily in this space, driven by compelling data and the enormous market potential. The dream of targeting aging itself, rather than its individual symptoms, is inching closer to reality. These breakthroughs in senolytic targeting are not just about adding years to life, but more importantly, about adding life to years, promising a future where old age can be synonymous with health and vitality.

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025